January marks Cervical Cancer Awareness Month 📅
IDEVAX engages in raising awareness on the fight against cervical cancer affecting millions of women worldwide and the importance of vaccines in prevention and treatment. Cervical cancer is still the fourth most common type of cancer in women, and more than 95% of cervical cancer is caused by the Human Papilloma Virus (HPV).
Prophylactic HPV vaccines
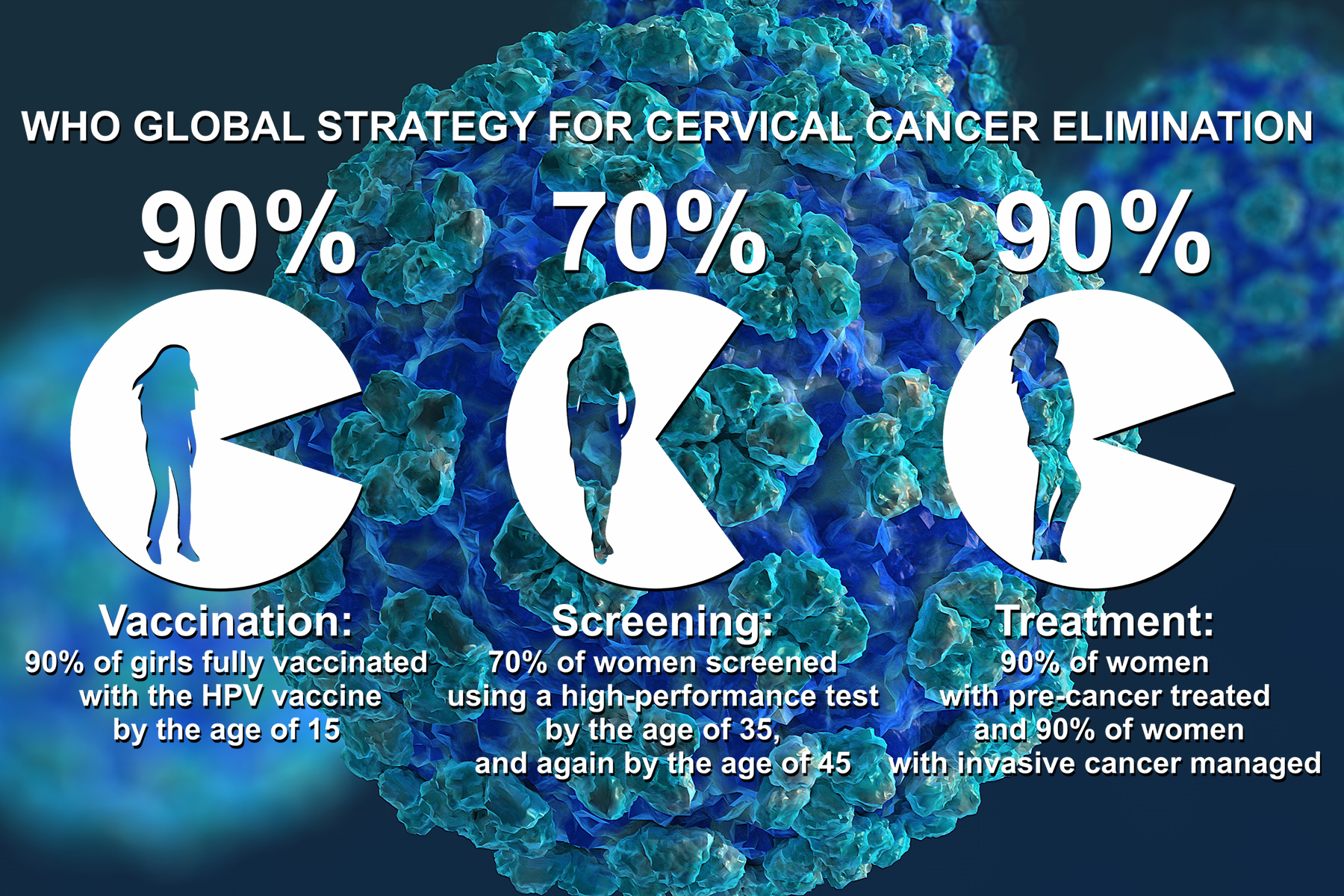
Prophylactic vaccination is a fundamental pillar of the WHO Global Strategy to Accelerate the Elimination of Cervical Cancer.
Since 2006, three types of prophylactic HPV vaccines based on virus-like particles (VLP’s) assembled from recombinant HPV capsid proteins (bivalent, tetravalent, and 9-valent), have been approved [1].

HPV Vaccination in LMIC
At the end of 2019, 88% of high-income countries introduced HPV vaccination in women and girls compared to less than 40% of low- and middle-income countries (LMIC). Additionally, 44% of high-income countries also started vaccinating boys, compared to only 5% of LMICs [1].
Unfortunately, between 2019 and 2021, coverage of the first dose of vaccination fell to 15%, representing a 25% drop. As a result, 3.5 million more girls missed out on HPV vaccination in 2021 compared to 2019.
WHO recommendations on HPV vaccination
Importantly, WHO recently updated its recommendations for prophylactic HPV vaccination to improve global access [2,3]:
- A one or two-dose schedule for girls aged 9-14 years
- A one or two-dose schedule for girls and women aged 15-20 years
- Two doses with a 6-month interval for women older than 21 years
Furthermore, vaccination of secondary target populations (females >= 15 years, boys, older males or MSM) is only recommended when this is feasible or affordable and does not divert resources from vaccination of the primary target population (girls aged 9-14 years).
Therapeutic HPV vaccines
Next to prophylactic HPV vaccines there is an urgent need to develop therapeutic vaccines to eradicate existing HPV infections and associated diseases.
Interestingly, promising results have been observed with clinical trials involving therapeutic HPV vaccines to treat established cervical cancer [1].
Want to know more?
Want to know more on HPV related cancers and vaccination? Read our educational white paper on prophylactic and therapeutic vaccines against HPV.
👉 Link to the white paper
References
- Rbeihat et al. 2023 New insights into the potential of prophylactic and therapeutic intradermal vaccination against Human Papilloma Virus (HPV)
- World Health Organization. (n.d.). WHO updates recommendations on HPV vaccination schedule. Retrieved January 19, 2023, from https://www.who.int/news/item/20-12-2022-WHO-updates-recommendations-on-HPV-vaccination-schedule
- World Health Organization. (n.d.). Human papillomavirus vaccines: WHO position paper, December 2022. Retrieved January 19, 2023, from https://www.who.int/publications/i/item/who-wer9750
Interested in our solutions?
Contact our commercial team!
