Immunotherapy: A solution to cure prostate cancer?
With an estimated almost 1.4 million new cases and 375,000 deaths worldwide, prostate cancer is the second most frequent cancer and the fifth leading cause of cancer death among men in 2020 [1].

In its early stages, prostate cancer is highly treatable with surgery and radiotherapy, with five-year survival rates close to 100% but once prostate cancer (PCa) has metastasized, the 5-year survival rate is reduced to less than 30% [2]. For patients with metastatic prostate cancer, androgen deprivation therapy is the standard treatment. However, as the tumors become resistant, most of these patients progress into metastatic castration resistant prostate cancer (mCRPC) and eventually die after progression [3]. As a result, new therapeutic approaches are needed to decrease the mortality rate of metastatic castration resistant prostate cancer.
Immunotherapy a breakthrough treatment for many cancers subtypes
In recent years, cancer immunotherapy, or the use of the body’s immune system to attack tumor cells, has received recognition as a viable cancer treatment strategy [4].
There are two principal ways to enhance the immune system’s antitumor activity.
- Blocking the immune-suppressive signals responsible for the decreased antitumor response.
In this case immune checkpoint inhibitors (ICIs) block checkpoint proteins that stop the immune system from attacking the cancer cells. - Stimulating the immune activation of antigen-presenting cells against specific tumor-associated antigens (TAAs) [5].
Although a variety of cells can function as antigen-presenting cells, three cell types are classified as “professional” antigen-presenting cells: dendritic cells, macrophages and B cells.
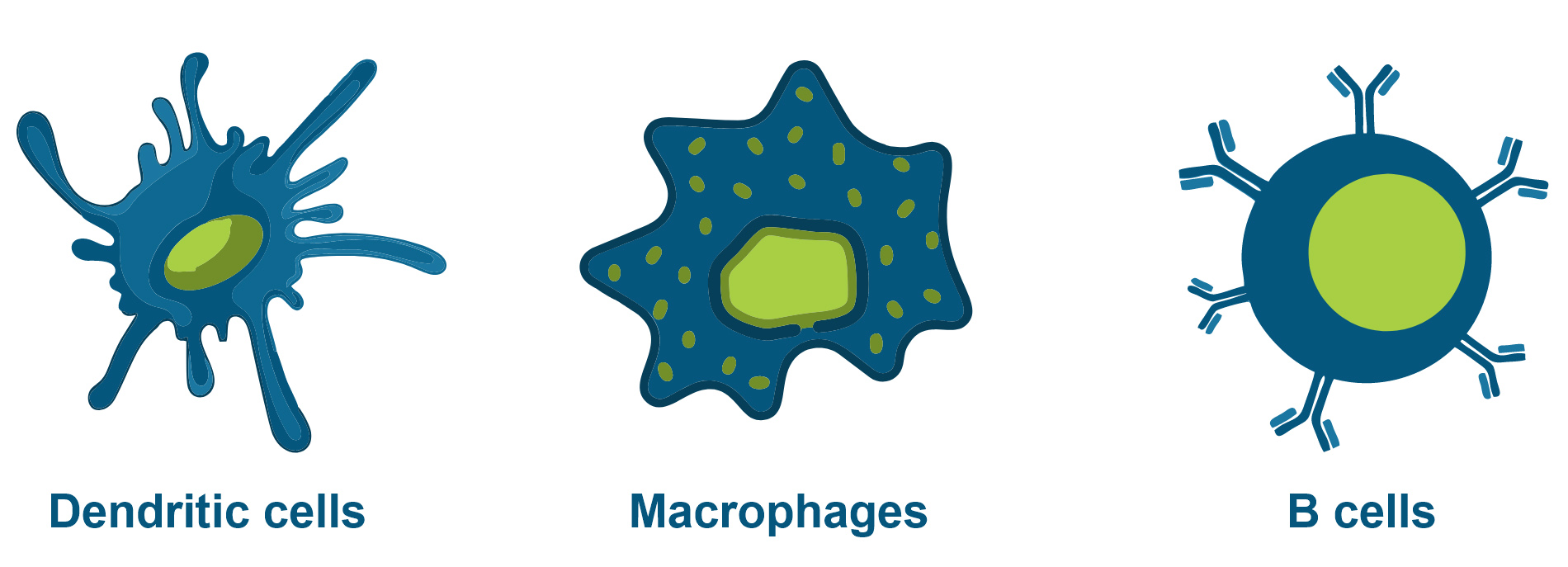
Today, most cancer vaccines are therapeutic vaccines that aim to clear active disease instead of preventing disease. The mechanism relies on the effective activation of dendritic cells (DCs), the most potent antigen-presenting cells (APCs), and the subsequent initiation of native T cell responses and boosting of cytotoxic T cell responses [4].
Next to therapeutic vaccines, there are a few vaccines available that aim at preventing cancers:
(1) cancers caused by Human Papilloma Virus (HPV), like cervical cancer and head & neck cancer [6]. (2) cancers caused by Hepatitis B virus (HBV), the most common etiologic agent of hepatocellular carcinoma (HCC), which is one of the five leading causes of cancer death in humans [7].
Interestingly, the first two cancer vaccines with therapeutic use were approved for genitourinary (GU) malignancies: Bacillus Calmette–Guerin (BCG) for non-muscle invasive bladder cancer (NMIBC) and Sipuleucel-T (Provenge®) for metastatic castration-resistant prostate cancer (mCRPC) [5].
Immunotherapy as a treatment option for Prostate Cancer
Given its high targetable number of TAAs that can be recognized, taken-up, processed and presented by antigen-presenting cells (APCs) to naïve T-cells, PCa is an ideal candidate for vaccine therapies. These TAA’s are predominantly expressed in prostate tissue [5,8].
- Prostatic Acid Phosphatase (PAP)
- Prostate Specific Antigen (PSA)
- Prostate-Specific Membrane Antigen (PSMA)
- Prostate Stem Cell Antigen (PSCA)
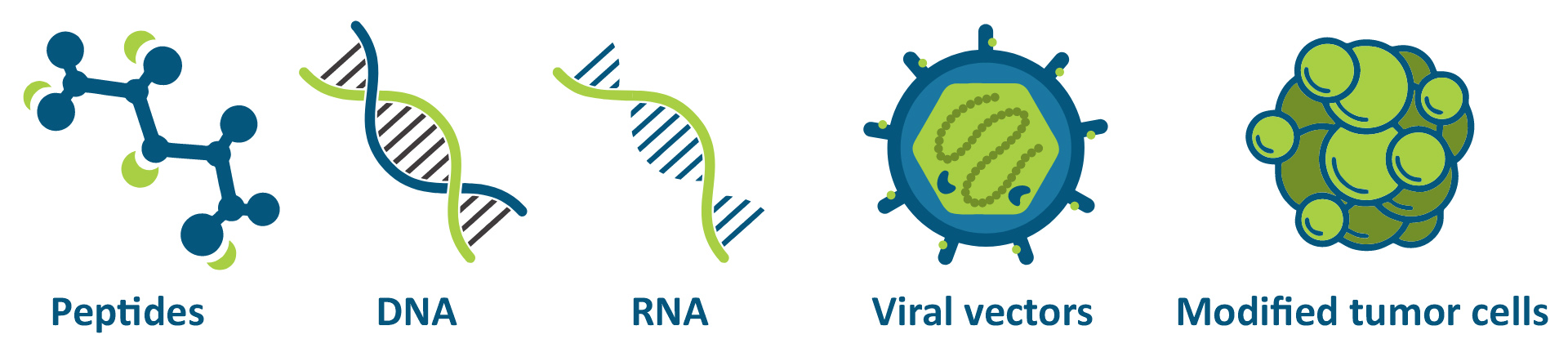
There are different ways to present TAAs to the immune system: DNA and RNA encoding TAAs, peptides, viral vectors and modified tumor cells [5].
FDA approved the first and only prostate cancer vaccine, Sipuleucel-T (Provenge®), in 2010. Unfortunately, despite being the first cancer with approved cancer vaccine therapy, results of immunotherapy in prostate cancer so far have been disappointing compared to some other types of cancer [3].
Fortunately, researchers and vaccine developers are not giving up, as of July 2020, there are thirteen vaccine clinical trials actively recruiting patients with prostate cancer [8].
mRNA vaccines: a more potent and versatile cancer vaccine platform?
More than two decades ago, the first proof-of-concept- and feasibility studies regarding RNA cancer vaccines were published. Since then, numerous preclinical and clinical studies have demonstrated the viability of mRNA vaccines to combat cancer [8].
Compared to other approved cancer vaccines, oncology mRNA vaccines may be more versatile, pragmatic, affordable, and effective [9]. Especially in the field of oncology, advantages such as rapid and versatile development, convenient modular design, and entirely cell-free synthesis, are being progressively recognized.
There are different routes for therapeutic mRNA vaccine delivery that may impact the induced immune response [9]. As a variety of antigen-presenting cells reside in the skin, the intradermal route of delivery has been widely used for mRNA cancer vaccines [8]. Compared to other types of vaccination, intradermal injection provokes indeed an improved immune response [10]. This superior immune response may even reduce the need for adjuvants for some vaccines [11].
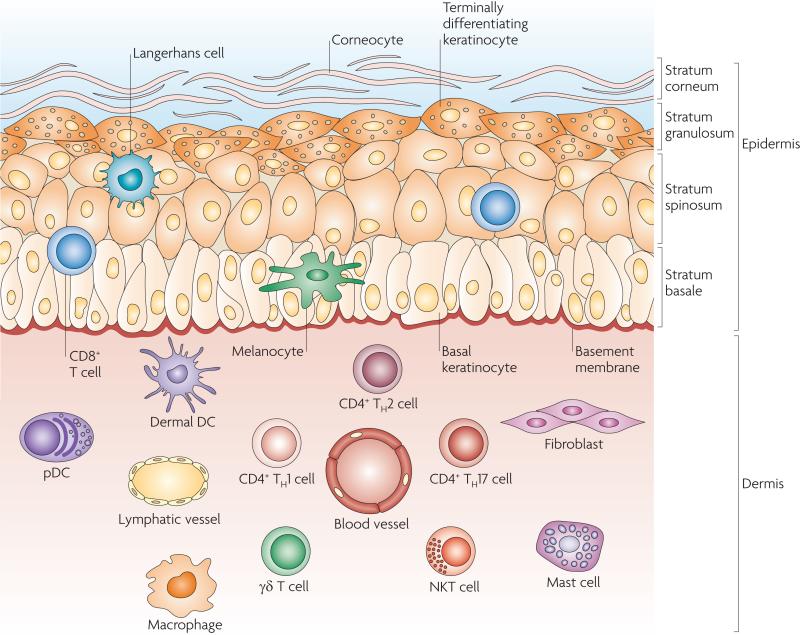
Skin Immune Network [12]
Opposite to the intradermal route of administration, mildly toxic vaccine adjuvants are often added to vaccines as a way to boost the immune response and are considered appropriate for seasonal applications, but when repurposed for continual oncology usage, toxicity may be problematic [9].
There is a clear need to explore more “patient-friendly” cancer vaccines and/or administration routes
VAX-ID® offers a unique and user-friendly intradermal injection system that allows for accurate and reliable intradermal delivery of both prophylactic and therapeutic vaccines.

Want to learn why VAX-ID® has the potential to increase the efficacy of immune therapies and patient comfort for (prostate) cancer treatment? Contact us!
References:
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660 (PMID: 33538338)
2. Immunotherapy for prostate cancer. Cancer Research Institute. (2022, October 17). Retrieved November 17, 2022, from https://www.cancerresearch.org/cancer-types/prostate-cancer
3. Šamija I, Fröbe A. CHALLENGES IN MANIPULATING IMMUNE SYSTEM TO TREAT PROSTATE CANCER. Acta Clin Croat. 2019;58(Suppl 2):76-81. doi:10.20471/acc.2019.58.s2.13 (PMID: 34975203)
4. Nguyen TT, Nguyen TTD, Ta QTH, Vo VG. Advances in non and minimal-invasive transcutaneous delivery of immunotherapy for cancer treatment. Biomed Pharmacother. 2020;131:110753. doi:10.1016/j.biopha.2020.110753 (PMID 33152919)
5. Maiorano BA, Schinzari G, Ciardiello D, et al. Cancer Vaccines for Genitourinary Tumors: Recent Progresses and Future Possibilities. Vaccines (Basel). 2021;9(6):623. Published 2021 Jun 9. doi:10.3390/vaccines9060623 (PMID: 34207536)
6. Paston SJ, Brentville VA, Symonds P, Durrant LG. Cancer Vaccines, Adjuvants, and Delivery Systems. Front Immunol. 2021;12:627932. Published 2021 Mar 30. doi:10.3389/fimmu.2021.627932 (PMID: 33859638)
7. Chang MH. Prevention of Hepatitis B Virus Infection and Liver Cancer. Recent Results Cancer Res. 2021;217:71-90. doi:10.1007/978-3-030-57362-1_4 (PMID: 33200362)
8. Bansal D, Reimers MA, Knoche EM, Pachynski RK. Immunotherapy and Immunotherapy Combinations in Metastatic Castration-Resistant Prostate Cancer. Cancers (Basel). 2021;13(2):334. Published 2021 Jan 18. doi:10.3390/cancers13020334 (PMID: 33477569)
9. Tsao SY. Potential of mRNA vaccines to become versatile cancer vaccines. World J Clin Oncol. 2022;13(8):663-674. doi:10.5306/wjco.v13.i8.663 (PMID: 36160466)
10. Zehrung D, Jarrahian C, Wales A. Intradermal delivery for vaccine dose sparing: overview of current issues. Vaccine. 2013;31(34):3392-3395. doi:10.1016/j.vaccine.2012.11.021 (PMID: 23176978)
11. Tsals I. Usability evaluation of intradermal adapters (IDA). Vaccine. 2017;35(14):1797-1801. doi:10.1016/j.vaccine.2016.07.036 (PMID: 27496277)
12. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679-691. doi:10.1038/nri2622 (PMID: 19763149)
Interested in our solutions?
Contact our commercial team!
