Photo by Fusion Medical Animation on Unsplash.
Sars-Cov-2 virus (COVID-19) and vaccination
In the last two years, the world has changed significantly due to the emergence of the Sars-Cov-2 virus, entering the world into a global pandemic. Novel and effective vaccines have been developed at an unprecedented speed thanks to hard work and tight cooperation between researchers, manufacturers and authorities. Nevertheless, the proportion initially mentioned by The World Health Organization (WHO) of 60-70% vaccination coverage needed to reach herd immunity against COVID-19 [1], has not yet been achieved. A huge discrepancy in vaccination coverage (ranging from 1% to over 70%) between the Western and Low- and Middle-Income countries have impacted the uptake of the vaccines. That being so, the WHO has set the 70% vaccinated global population target to the middle of 2022 [2].
Routes of Administration
Different routes of administration of vaccines, like intradermal (ID) vaccination whereby vaccines are injected in the skin instead of the muscle (intramuscular, IM) could offer solutions in global vaccine availability. Indeed, ID vaccination has been praised for its dose-sparing potential as it can save up to 10 times the vaccine dose, decrease volumes in the cold chain, and increase acceptability [3].
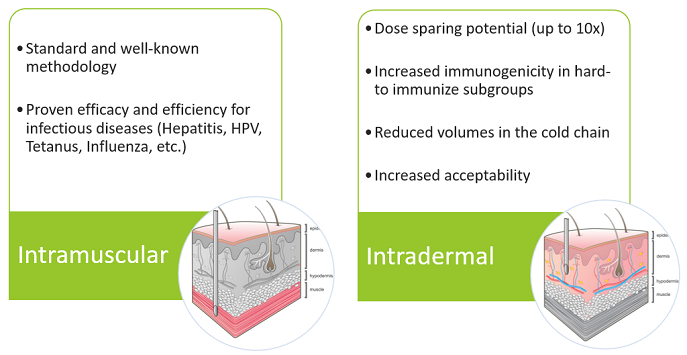
Intradermal vaccination
The Italian Society of Mesotherapy published earlier this year a narrative review of scientific literature on the efficacy of fractional ID vaccination in comparison with full doses and raised the question if ID vaccinations can serve in the battle against the COVID-19 pandemic [4]. Overall, the findings demonstrated the promising potential of ID injections for vaccination regarding important aspects such as vaccine efficacy and efficiency, and dose-sparing capability. As a matter of fact, several studies have been performed for investigating the safety and efficacy of ID delivery vaccines [5]. Accordingly, the society alerted the scientific community and health authorities for the urgency to investigate the potential of fractional ID administration for anti-COVID-19 vaccination.
Intradermal vaccination against Sars-Cov-2
The Idevax team has recently conducted a literature review covering the last 5 years specifically on ID vaccines on PubMed using the search queries: “intradermal vaccination” OR “ID vaccination” OR “dermal vaccination”. We found a considerable increase in the number of publications over the last 5 years in this field and a total of 111 new publications surged. Studies with mRNA vaccines, the technology used by Pfizer (Comirnaty®) and Moderna (Spikevax®), showed to be well tolerated and provided a robust immune response [6]. Furthermore, using the European and American clinical trial databases (EU Clinical Trials Register, ClinicalTrials.gov) we have identified 7 ongoing trials investigating the ID route for COVID-19 vaccination and most should be concluded early next year.
Dose-sparing potential of intradermal vaccination
A recently conducted randomised clinical trial in the Netherlands evaluated the safety and immunogenicity of fractional intradermal doses of the marketed COVID-19 vaccine mRNA-1273 (Moderna) [7]. The results showed that the fractional ID doses, that were 10% and 20% of the standard IM dose, was safe and capable of inducing a robust antibody response with superior or equivalent antibody titers. These exciting findings suggest that a dose-sparing strategy using anti COVID-19 vaccines, especially the mRNA-vaccine could provide protective immunity and could offer solutions to accelerate COVID-19 vaccination campaigns across the world.
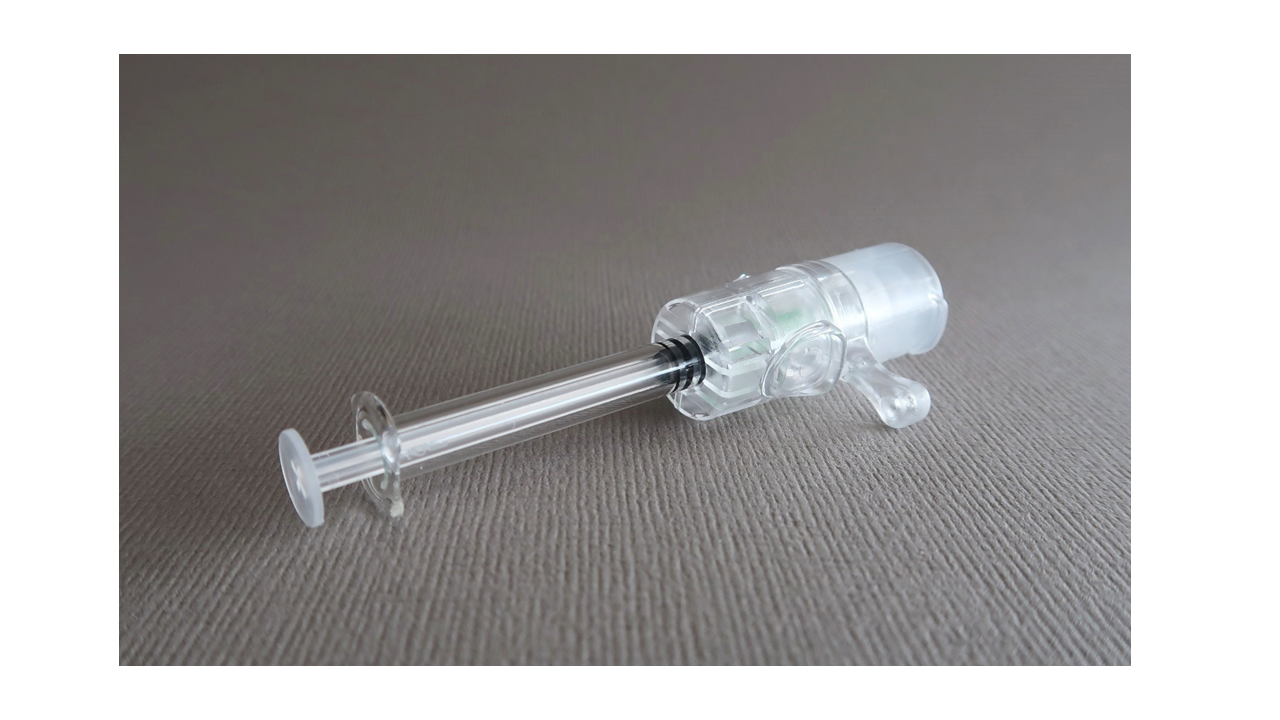
VAX-ID® | An injection device suited for accurate intradermal vaccination
VAX-ID® by IDEVAX is an innovative medical device, a needle adaptor, which is in development to perform accurate intradermal injections. The device has shown well-suited for Hepatitis B booster vaccination as the ID route elicited a non-inferior immune response with 1/4th of the dose compared to IM [8]. VAX-ID® can be used with marketed liquid-formulated vaccines thereby offering a solution for accurate ID injections for COVID-19 vaccines.
References:
- Covid-19: How Much Herd Immunity is Enough? – The New York Times. <https://www.nytimes.com/2020/12/24/health/herd-immunity-covid-coronavirus.html> (accessed on 16 Dec 2021).
- WHO, UN set out steps to meet world COVID vaccination targets. October 2021. <https://www.who.int/news/item/07-10-2021-who-un-set-out-steps-to-meet-world-covid-vaccination-targets> (accessed on 16 Dec 2021).
- Vankerckhoven & Van Damme, Clinical studies assessing immunogenicity and safety of intradermally administered influenza vaccines. Exp Onion 2010.
- Migliore A. et al. Intradermal Vaccination: A Potential Tool in the Battle Against the COVID-19 Pandemic? Risk Manag Healthc Policy. 2021 May 20; 14:2079-2087. doi: 10.2147/RMHP.S309707. PMID: 34045909; PMCID: PMC8144901.
- Schnyder JL. et al. Fractional dose of intradermal compared to intramuscular and subcutaneous vaccination – A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Sep-Oct;37:101868. doi: 10.1016/j.tmaid.2020.101868. Epub 2020 Sep 6. PMID: 32898704; PMCID: PMC7474844.
- Feldman RA. et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019 May 31;37(25):3326-3334. doi: 10.1016/j.vaccine.2019.04.074. Epub 2019 May 10. PMID: 31079849.
- Geert V.T.R. et al. Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA-1273 SARS-CoV-2 vaccine in healthy adults as a dose sparing strategy. 2021 Jul 27;21261116. MedRxiv; doi: 10.1101/2021.07.27.21261116/.
- Van Mulder TJM et al. Immunogenicity and safety of intradermal delivery of hepatitis B booster vaccine using the novel drug delivery device VAX-ID™. Vaccine 2019 Jan 21; 37(4):581-586.
Interested in our solutions?
Contact our commercial team!
