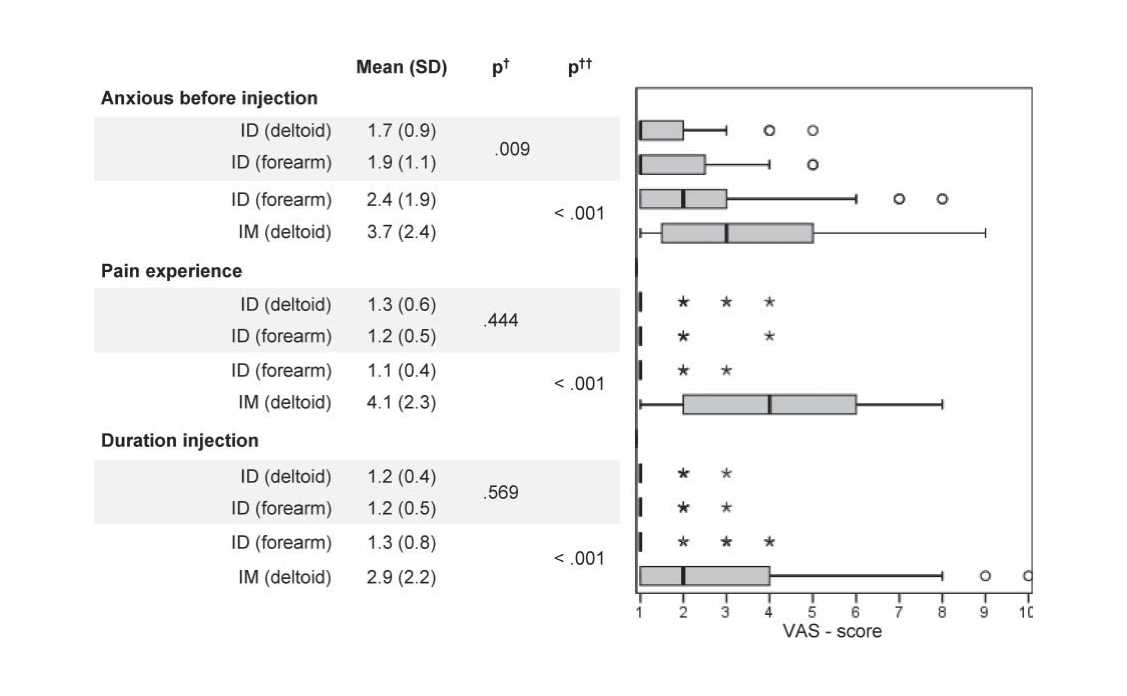
Acceptability and usability of VAX-ID was assessed in 102 healthy volunteers aged 18 to 65 years old. To compare injection site and route of administration, subjects were allocated to 4 subgroups, either receiving subsequently 2 intradermal (ID) injections (one in the forearm and one in the deltoid) or an ID (forearm) and an intramuscular (IM) (deltoid) injection. All injections contained saline solution.
Results showed that anxiety before injection, pain during injection and duration of injection were rated significantly lower for ID compared to IM. One day after the injections, redness was reported more often after ID injection in the forearm versus ID in the deltoid; pain at injection site was reported significantly more often after IM vs. ID injection.
The new VAX-ID prototype device was found easy to handle, easy to use and safe. The new VAX-ID prototype device was shown to have a high degree of acceptability as well as usability.
Interested in our solutions?
Contact our commercial team!
