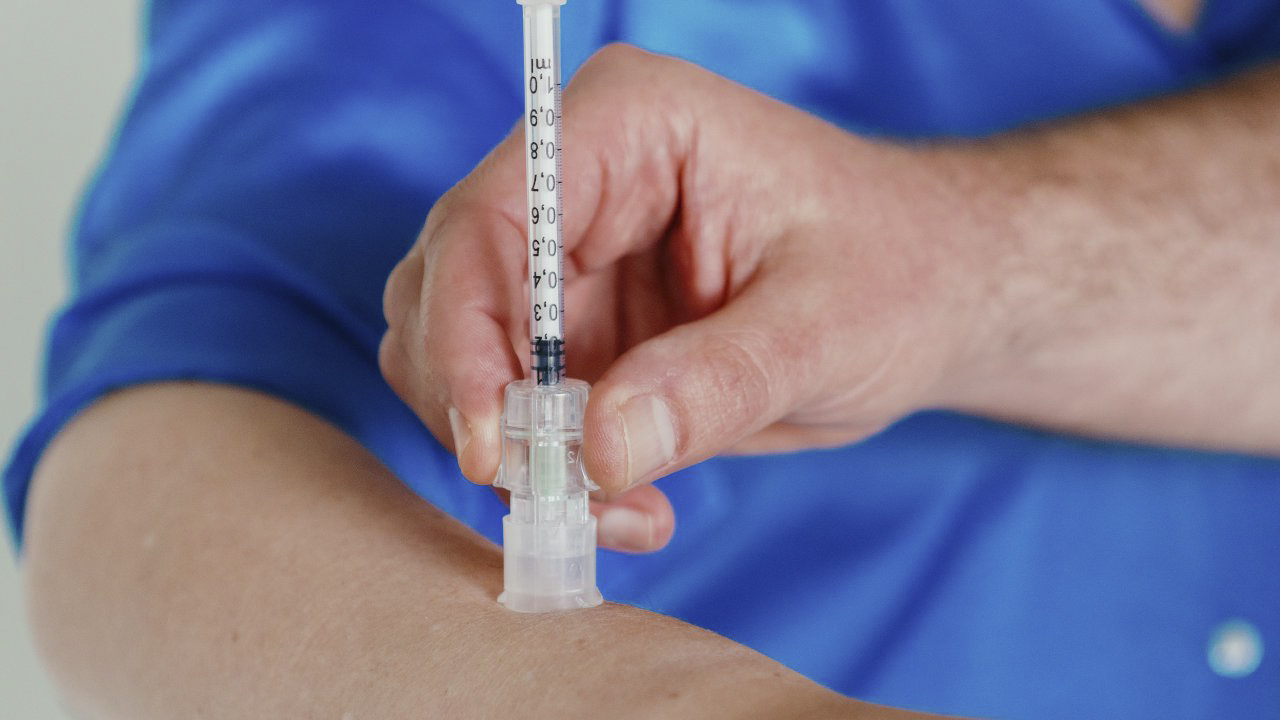
Our skin – an overlooked optimal site for skin drug delivery and vaccination
Skin drug delivery or intradermal injection (i.e. injection in the dermal layer of the skin) offers great benefits for both prophylactic and therapeutic vaccines. Why? The skin is our first line of defense offering a dense network of immune cells. Indeed, for prophylactic vaccines there is a clear dose-sparing potential as only 1/10th of the dose is needed compared to intramuscular and subcutaneous administration. Also, the rich immune network offers beneficial benefits for therapeutic drug delivery, including cell therapy and cancer immunotherapy.
Our solution – VAX-ID
VAX-ID® is the heart of Idevax, an ISO13485:2016 certified company. It is an award-winning patented drug delivery device suited for reliable intradermal injection with high ease of use.
VAX-ID® is CE marked as a Class IIa medical device, meeting the stringent European Medical Device Regulations for products sold within the European Economic Area (EEA). For other regions, devices are currently available for investigational use only (IUO) and research use only (RUO).